Nanomaterials with dual immunomodulatory functions for synergistic therapy of breast cancer brain metastases
Time:2023/6/28 15:08:20 Views:573
A long-standing paucity of effective therapies results in the poor
outcomes of triple-negative breast cancer brain metastases. Immunotherapy has
made progress in the treatment of tumors, but limited by the non-immunogenicity
of tumors and strong immunosuppressive environment, patients with TNBC brain
metastases have not yet benefited from immunotherapy. Dual immunoregulatory
strategies with enhanced immune activation and reversal of the
immunosuppressive microenvironment provide new therapeutic options for
patients. Here, we propose a cocktail-like therapeutic strategy of
microenvironment regulation-chemotherapy-immune synergistic sensitization and
construct reduction-sensitive immune microenvironment regulation nanomaterials.
The related results, titled nanomaterials with dual immunomodulatory functions
for synergistic therapy of breast cancer brain metastases, were published
online in the Bioactive Materials, an internationally renowned journal.
Brain
metastases (BM) are the most common type of endogenous brain tumors, occurring
in approximately 30% of patients with metastatic breast cancer (BC). Among BCs,
up to 46% of patients with triple-negative breast cancer (TNBC) will develop
brain metastases and exhibit the worst therapeutic outcomes because of the
paucity of effective therapies (the median survival is only 4.9 month). The
treatment of TNBC brain metastases is largely palliative, but neither
conventional chemoradiotherapy nor surgery has achieved obvious curative effect
due to the existence of blood-brain barrier (BBB) and multiple metastases.
There is currently no effective clinical treatment and it is urgent to find new
treatment strategies.
Due to the
potential of the immune system among various novel therapeutic strategies,
immunotherapy has drawn the attention of researchers. Immune-based strategies,
however, have been limited to date by the traditional notion that BC is
immunologically cold or minimally immunogenic. Approaches to reprogram tumor
microenvironment (TME) to convert cold tumors into hot tumors and thus improve
the efficacy of immunotherapy are gradually being developed. Of concern,
however, is that the central nervous system (CNS) is traditionally considered
an immune privileged site without most peripheral immune cells. Combined with
limited penetration of conventional drugs into the brain, patients with BMs are
excluded from many clinical trials involving immunotherapies (IT), limiting
current data related to IT for BCBM treatment]. While there is clear evidence
that T cells do infiltrate BCBMs and lower the accumulation of tumor
infiltrating lymphocytes (TILs) in BM. Besides activation of the immune
response via immunogenic cell death (ICD) prolongs survival in mice with brain
metastases, suggesting that enhance T cell trafficking to BCBMs may be a valid
strategy for enhancing efficacy.
While the
presence of TILs is often correlated with better prognosis and indicates higher
response rates to immunotherapy, the presence of immunosuppressive components in
TME is associated with tumor promotion and therapy resistance. Establishment of
the brain micrometastases requires reactive, inflammatory components including
early infiltration and reprogramming of various immune cells and astrocytes
within the brain as a “pre-metastatic niche”. Signal transducer and activator
of transcription 3 (STAT3) is abnormally activated in tumor cells and
astrocytes of BCBM, and promotes the synthesis and secretion of downstream
cancer-promoting signal molecules via intercellular signaling, thus inducing
the M2 polarization of tumor-associated macrophages (TAM), inhibiting the
infiltration of CD8+ T cells, and forming the immunosuppressive metastatic
microenvironment. Silibinin (SIL), a STAT3 inhibitor targeting these signaling
axes in BM, has been shown to improve BCBM outcomes. Microenvironmental
regulatory strategies to reverse immunosuppression by inhibiting STAT3
phosphorylation therefore represent an intriguing target for BM treatment.
In this study, based on the cocktail-like strategy for
microenvironment regulation-chemotherapy-immune synergistic sensitization
involving BBB targeting and concentrated drug release within BCBMs, we
constructed nanomaterials (SIL@T) with dual immunomodulatory functions to
deliver SIL for reversing the immunosuppressive microenvironment and
Oxaliplatin (OXA) for increasing the infiltration of TILs. Anchored with the
CSKC optimized by ligandanalogs of insulin-like growth factor 1 receptor
(IGF-1R), SIL@T can penetrate the BBB and subsequently target the brain
metastases. Upon internalization by metastatic tumor cells, the micellar
structure was destroyed, the encapsulated SIL was leaked to inhibit STAT3
phosphorylation, and OXA was released responsively to induce ICD in the highly
reduced cytoplasmic environment. The pharmacodynamic results in the TNBC brain
metastasis model mice (BM-mice) also showed that SIL@T could stimulate
dendritic cells (DC) maturation and increase CD8+ T cell infiltration in the
metastatic area, while inhibiting the activation of STAT3 in metastatic cells
to reverse the immunosuppressive metastatic microenvironment, significantly
prolonging the survival time of BM-mice.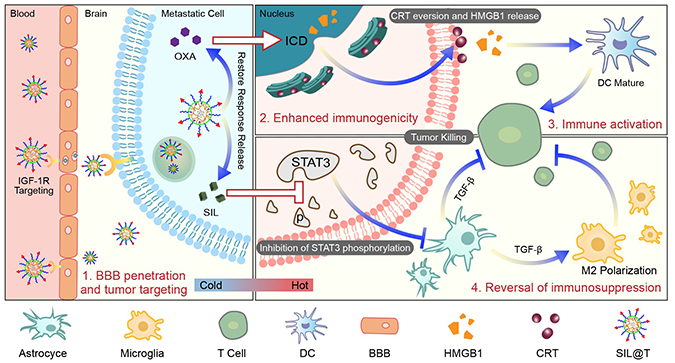
Doctoral student Zhao Zhenhao in our group is the first authors of
the paper, and Professor Jiang Chen is the corresponding author of the paper.
The research was supported by the National Natural Science Foundation of China,
the Shanghai Academic Research Leaders Program and other projects.