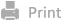Disrupting Tumor Lactate Homeostasis to Sensitize Chemo-Immunotherapy Using a Glucose-Disguised Lactate Interceptor
Time:2025/6/9 10:45:01 Views:1846
Aberrantly elevated lactate flux in tumors is increasingly recognized as a key driver of metabolic symbiosis, immunosuppression, and ultimately, immunogenic chemotherapy resistance. Here, we propose a precise lactate homeostasis modulation strategy that selectively intercepts intracellular lactate molecules in highly glycolytic tumor cells. Targeting monocarboxylate transporter 4 (MCT4), a key lactate efflux transporter overexpressed in tumor cells, we developed a glucose-disguised delivery system for precise transport of regulatory molecules into glycolysis-dependent tumor cells. By modulating lactate-mediated crosstalk between heterogeneous tumor subpopulations (glycolysis-dependent and lactate-consuming cells) and immune cells, this strategy effectively disrupts lactate-driven metabolic cooperation within the tumor niche, which may contribute to overcoming lactate-associated resistance to chemo-immunotherapy.
The related results, titled Disrupting Tumor Lactate Homeostasis to Sensitize Chemo-Immunotherapy Using a Glucose-Disguised Lactate Interceptor, were published online in the ACS Nano (IF=15.8).
Chemo-immunotherapy can induce immunogenic cell death (ICD) in the tumor, offering therapeutic benefits beyond anti-proliferation. However, the proliferative mechanisms and immune evasion driven by tumor metabolism often limit its clinical efficacy.
A growing body of evidence suggests that lactate plays a crucial role as a stabilizing agent in maintaining high metabolic flux and reinforcing the immunosuppressive tumor microenvironment. Glycolytic tumor cells produce large amounts of lactate, while oxidative tumor cells utilize lactate as a primary energy source, thus forming an efficient metabolic chain that supports tumor proliferation. Lactate not only acidifies the tumor microenvironment but also modifies proteins via lactylation, thereby reducing tumor immunogenicity and suppressing the metabolism and function of various immune cells. Given its central role in tumor progression, intervening in intracellular and intercellular lactate homeostasis has emerged as a promising strategy to enhance chemo-immunotherapy. Current lactate metabolism-targeting drugs predominantly inhibit lactate dehydrogenase (LDH), but the widespread expression of LDH across various cell types often leads to off-target effects, thereby limiting its clinical application.
As the sole channel for lactate flow, lactate transporters play a key role in maintaining lactate homeostasis. Notably, we noticed that monocarboxylate transporter 4 (MCT4) is significantly upregulated in glycolytic tumor cells, whereas oxidative tumor cells and immune cells predominantly express other types of transporters. This difference likely arises from the fact that glycolytic tumor cells, as the major lactate exporters, produce lactate at higher rates than surrounding cells. These cells heavily rely on MCT4 to maintain intracellular lactate balance and sustain the extracellular high-lactate environment. Thus, we hypothesize that targeting MCT4-dependent lactate export in glycolytic tumor cells could serve as a key mechanism to efficiently induce lactate homeostasis collapse. However, glycolytic tumor cells are predominantly located in hypoxic tumor regions, distant from blood vessels, making targeted drug delivery to these areas a significant challenge. Additionally, there is currently a lack of effective MCT4 inhibitors, further complicating therapeutic intervention.
Inspired by the metabolic characteristics of glycolytic tumor cells, particularly their elevated glucose transporter expression, we developed a glucose-disguised lactate interceptor (GDLAI) targeting MCT4. This system incorporates MCT4-targeting siRNA to achieve precise and efficient gene silencing; the glucose-modified cationic dendritic lysine (DGL) enables targeted co-delivery of siRNA and chemotherapy drugs to hypoxic cells. Using oxaliplatin prodrug as a model compound, GDLAI not only inhibits tumor proliferation but also induces ICD. These three components self-assemble in aqueous solutions, forming stable nanoparticles. In the colorectal cancer (CRC) model, we demonstrate that GDLAI can specifically deliver metabolic modulators to glycolytic tumor cells, with potential implications for lactate homeostasis modulation and chemo-immunotherapy sensitization (Scheme 1).

Scheme 1. Schematic illustration of the strategy to disrupt lactate homeostasis using the glucose-disguised lactate interceptor (GDLAI) to enhance chemo-immunotherapy, aiming to inhibit tumor proliferation and strengthen the anti-tumor immune response.
Xuwen Li, a doctoral student (member of the Zhuo Bo Program) in our research group, is the first author of the paper, while Professor Chen Jiang is the corresponding author. This study has received support from projects such as the National Natural Science Foundation of China and the Shanghai Academic Research Leaders Program and other projects.
Original link: https://pubs.acs.org/doi/10.1021/acsnano.5c03545