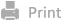Size and Charge Dual-switchable Nanoparticles for Achieving Chemo-sensitization and Immune Infiltration Against Pancreatic Ductal Adenocarcinoma
Time:2024/12/3 15:46:54 Views:534
Pancreatic ductal adenocarcinoma (PDAC) is a gland-forming malignant tumor that originates from the pancreas, a retroperitoneal organ. Its incidence is low but has been increasing in recent years, with 13 cases per 100,000 individuals per year and a lifetime risk of less than 2%, without sex or geographic bias. Pancreatic adenocarcinoma is usually diagnosed at an advanced stage of development, resulting in low overall and progression-free survival. Ninety percent of these cases correspond to the PDAC subtype. The goal of the current study was to convert the crosstalk between tumor cells and the tumor microenvironment into promising therapeutic solutions. The possibility of identifying specific targeted pathways in certain patient subpopulations allows us to personalize treatment and improve outcomes. The clinical first-line chemotherapeutic agent gemcitabine, which is most commonly used for PDAC, has only a 10–20% beneficial clinical response. A major factor in chemoresistance is the dense fibrotic network formed by the intertwining of the extracellular matrix (ECM), which protects tumor cells. Cancer cells upregulate the expression of collagen family proteins in a paracrine manner by stimulating fibroblasts. ECM proteins, including collagen and hyaluronic acid, are deposited as a result of the connective tissue proliferative response, which creates high interstitial fluid pressure and results in low vascular density within the tumor. The dense stromal barrier represents not only a physical barrier but also a bio-functional barrier, restricting the proper diffusion of antitumor active agents and hindering the infiltration of immune cells; thus, the stromal barrier plays a key role in PDAC progression and treatment. In recent years, research using nanomaterials for drug delivery to PDAC has increased, and many of the advantages of nano-therapy have been identified, with well-known benefits including controlled drug release, prolonged circulation, attenuated side effects, and the ability to enable the co-delivery of multiple therapeutic agents.
Many therapeutic options have been developed for PDAC stroma, and research has focused on the destruction of the stromal barrier. When the stromal barrier is destroyed, chemotherapeutic agents can approach the tumor more easily and kill the tumor cells; however, blind ablation of the stromal barrier often leads to undesirable consequences, such as tumor metastasis. Moreover, simply destroying the stromal barrier cannot remodel the pancreatic tumor microenvironment. Among the ECM-interacting proteins, the discoidin domain receptor 1 (DDR1) is most prominently associated with tumor progression and drug resistance. It belongs to a subfamily of receptor tyrosine kinases, characterized by extracellular discoidin domains that regulate cell proliferation and differentiation by interacting with several types of collagen, such as collagen type I. When DDR1 is activated, it is transduced by DDR1-NF-κB-p62-NRF2 signaling to promote PDAC growth. Studies have shown that gemcitabine-induced oxidative stress is often accompanied by an increase in antioxidant responses, including increased NRF2 expression, which sheds light on the rationale for gemcitabine-induced drug resistance in pancreatic cancer.. When DDR1 inhibitors are used to block this signaling axis, NRF2 is inhibited, the intracellular redox balance is disrupted, and the accumulation of excess reactive oxygen species (ROS) leads to mitochondrial dysfunction and initiates the apoptotic program, which restores the sensitivity of pancreatic cancer cells to gemcitabine. Simultaneously, it has been shown that DDR1 promotes the alignment of collagen fibers, thereby strengthening tumor defense against immune infiltration. When DDR1 is inhibited, collagen alignment is disrupted, attenuating immune rejection and inhibiting tumor growth in immunocompetent hosts. Therefore, in this study, we used dasatinib, a tyrosine kinase inhibitor, to inhibit the DDR1-NF-κB-NRF2 signaling axis, to restore gemcitabine chemo-killing, and to disrupt collagen alignment in the stroma in order to deregulate the immunosuppressive microenvironment of PDAC.

Scheme 1. A) Schematic illustration of the preparation of DGD/L@Apt. B) ①Schematic diagram of the process of change in formulation size and charge. ②Pathway inhibition in cancer cells leads to cancer cell apoptosis. ③Inhibition of DDR1 by the formulation leads to disorganization of collagen alignment. ④Stromal ablation promotes tumor cell killing by immune cell infiltration.
Owing to the physiological barriers that exist in pancreatic cancer, the process of nanoparticle penetration into the tumor must be considered when designing formulations. The most common strategy for facilitating penetration is to design nanoparticles for multi-stage release and use transcellular transcytosis. Multi-stage release typically involves large nanoparticles that facilitate extravasation from the leaky vascular system to the tumor region, which can then be transformed into smaller nanoparticles to increase their diffusion in the tumor mesenchyme when triggered by the tumor microenvironment. Transcytosis also occurs in various forms, among which positively charged nanomedicines can effectively induce adsorption-mediated transcytosis and significantly facilitate extravasation into solid tumors. However, abundant plasma proteins are rapidly absorbed onto the cationic surface of circulating nanoparticles upon intravenous administration, resulting in the formation of a protein corona and inducing a conditioning effect. Consequently, cationic nanomedicines are rapidly cleared from the bloodstream by mononuclear phagocytic systems. Therefore, in this study, we used a linker responsive to the hypoxic microenvironment of PDAC to crosslink positively charged dendrigraft-L-lysine (DGL). Gemcitabine was coupled to the surface of DGL through chemical bonding, the cavity of DGL was used to load the hydrophobic drug dasatinib, and the surface of the nanoparticles was charge-masked using a nucleic acid aptamer targeting tenascin-C, a protein that is abundantly present in the stroma of PDAC, so that the surface of the nanoparticles was negatively charged. Upon arrival of the nanoparticles at the pancreatic tumor site, the surface-located nucleic acid aptamer GBI-10 initially detaches, causing the surface charge of the delivery system to shift from negative to positive. With enhanced penetration capability, nanoparticles gradually encounter a hypoxic environment, where the cleavage of chemical bonds in the linking units leads to a reduction in particle size, enabling deeper penetration into pancreatic cancer tissues. Concurrently, gemcitabine and dasatinib are released, and the interplay between these two drugs reverses chemotherapy resistance in pancreatic cancer cells and enhances immune cell infiltration within the tumor tissue.
Haolin Song, the PhD candidate from the School of Pharmacy, Fudan University, is the first author. Professor Chen Jiang is the corresponding author of this paper. The work was supported by grants from the National Natural Science Foundation of China, National Key R&D Program of China, Shanghai Municipal Science and Technology Major Project, and ZJLab.
For more information:https://doi.org/10.1002/adfm.202411643