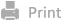Recent advances in biomimetic strategies for the immunotherapy of glioblastoma.
Time:2024/8/23 19:29:12 Views:781
Immunotherapy is regarded as one of the most promising approaches for treating tumors, with a multitude of immunotherapeutic thoughts currently under consideration for the lethal glioblastoma (GBM). However, issues with immunotherapeutic agents, such as limited in vivo stability, poor blood-brain barrier (BBB) penetration, insufficient GBM targeting, and represented monotherapy, have hindered the success of immunotherapeutic interventions. Moreover, even with the aid of conventional drug delivery systems, outcomes remain suboptimal. Biomimetic strategies seek to overcome these formidable drug delivery challenges by emulating nature's intelligent structures and functions. Leveraging the variety of biological structures and functions, biomimetic drug delivery systems afford a versatile platform with enhanced biocompatibility for the co-delivery of diverse immunotherapeutic agents. Moreover, their inherent capacity to traverse the BBB and home in on GBM holds promise for augmenting the efficacy of GBM immunotherapy. Thus, this review begins by revisiting the various thoughts on immunotherapy for GBM. Then, the barriers to successful GBM immunotherapy are analyzed and the corresponding biomimetic strategies are explored from the perspective of function and structure. Finally, the clinical translation's current state and prospects of biomimetic strategy are addressed. The related research were recently published online in the internationally renowned journal Biomaterials (Impact Factor = 12.8) under the title "Recent advances in biomimetic strategies for the immunotherapy of glioblastoma."

Scheme 1. Schematic diagram of biomimetic strategies for the immunotherapy of GBM.
Glioma represents the most prevalent primary malignant neoplasm of the central nervous system (CNS) and is notorious for its rapid progression and dismal prognosis. The World Health Organization (WHO) categorizes gliomas into four grades (I to IV) based on their pathological characteristics. Glioblastoma (GBM), designated as Grade IV, is the most formidable and intractable type, associated with a median survival of merely 4 to 15 months for affected individuals. Presently, the conventional therapeutic regimen for GBM consists of surgical intervention complemented by chemotherapy and/or radiation therapy (RT). Nevertheless, the invasive and infiltrative properties of GBM preclude distinct demarcation from healthy brain tissue, complicating the achievement of complete surgical excision. Consequently, residual tumor microfoci persist, precipitating the emergence of more aggressive recurrent tumors with even poorer prognoses. Additionally, the pronounced heterogeneity of GBM fosters drug resistance, thereby significantly undermining the efficacy of chemotherapeutic agents. These challenges underscore the critical need to develop more precise and efficacious therapies in the treatment arsenal for GBM.
In recent years, immunotherapy, which harnesses the body's immune system to attack tumors, has garnered significant achievements in combating aggressive cancers such as advanced melanoma and non-small cell lung cancer. Traditionally, CNS was regarded as "immune-privileged." However, a pivotal discovery in 2015 unveiled the existence of lymphatic vessels within the meninges [11]. Moreover, it was found that immune surveillance occurs around the dura mater sinuses, where CNS antigens are recognized and presented, leading to T cell recruitment and subsequent immune responses. This discovery indicates the presence of a distinct immune mechanism within the brain and establishes a basis for the immunotherapy of GBM. Further studies have demonstrated that GBM, akin to peripheral tumors, is a prototypical "cold" tumor immersed in a heavily immunosuppressive tumor microenvironment (TME). This environment is marked by the extensive infiltration of immunosuppressive cells and their secretions, along with a depletion of immune effector cells. Therefore, based on these characteristics of GBM, various immunotherapeutic thoughts have been developed, including enhancing the immunogenicity of GBM, reducing its immune evasion capabilities, and reprogramming immunosuppressive cells.
Despite various efforts, the efficacy of immunotherapy for GBM has not yet met expectations. The reason lies in that the realization of all thoughts actually depends on the action of immunotherapeutic agents within GBM lesions. However, most of these agents exhibit unfavorable physicochemical properties and are rapidly cleared by the body’s mononuclear phagocyte system upon systemic administration. Escalating dosages to overcome this can lead to potential adverse effects. Moreover, these drugs often fail to traverse the BBB and accumulate at the tumor site, resulting in suboptimal concentrations within the lesion. It is also notable that the majority of immunotherapeutic agents target specific cell types or pathways. Consequently, they typically induce a narrow spectrum of immune responses, which are often inadequate in the face of GBM’s complex TME. While conventional drug delivery systems can partly alleviate these challenges, their effectiveness remains constrained, and their efficiency is low. Furthermore, these systems are frequently encumbered by concerns over biocompatibility, off-target effects, and intricate formulation processes. Hence, there is an imperative need for innovative strategies to deliver a diverse array of immunotherapeutic agents safely and precisely to GBM lesions, thereby potentiating the success of immunotherapy in treating GBM.
Biomimetic strategies encompass the emulation of natural organisms’ intricate structures and functions, alongside the integration of biological principles with materials science and engineering, to tackle significant challenges in diverse fields. Within the biomedical sector, such strategies have catalyzed progress in tissue engineering, medical devices, and biosensing. On drug delivery, biomimetic concepts guide the construction of biomimetic drug delivery systems. These systems leverage the variety of biological structures and functions to offer distinct advantages, including heightened biocompatibility and innate capacity to traverse the BBB and target GBM. Moreover, they present a simple, easily scalable platform for the combined delivery of multiple drugs. Consequently, biomimetic strategies possess immense potential for the realization of effective immunotherapy for GBM. This review initially revisits the array of current immunotherapeutic thoughts for GBM comprehensively, subsequently analyzes the prevalent challenges immunotherapeutic agents encounter, and discusses the corresponding biomimetic strategies from the perspective of function and structure rather than material. Ultimately, we provide a perspective on the future of biomimetic strategies in GBM immunotherapy, with the aspiration that these endeavors will inspire novel developments in the immunotherapy of GBM.
Link to the original article: https://doi.org/10.1016/j.biomaterials.2024.122694