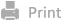Reconciling the Cooperative-Competitive Patterns Among Tumor and Immune Cells for Triple-Negative Breast Cancer Treatment Using Multimodule Nanocomplexes
Time:2024/4/23 14:58:14 Views:286
The intricate interplay between tumor cells,
neighboring immune cells, and various immune cell types involves complex
competitive-cooperative patterns rewired through metabolic and immune signals, which are essential for
shaping the immunosuppressive tumor microenvironment (TME). This metabolic
imbalance-immunosuppressive microenvironment plays a crucial role in
treatment-resistant triple-negative breast cancer (TNBC) by influencing the disease and
adverse prognoses. Malignant
proliferating tumor cells strategically reprogram their metabolism to sequester
essential nutrients from the TME and release various metabolites, thereby
influencing TME status. This imposition of metabolic stress on infiltrating
immune cells leads to alterations in metabolism-based functions. Recent
evidence has revealed that
tumor-associated macrophages (TAMs), which constitute
50% of the infiltrating immune cells in TNBC, are induced to upregulate
arginase-1 (Arg-1) under metabolic stress and polarize toward the
immunosuppressive M2 phenotype. Adding to the complexity, the tumor cells also
overexpress the antiphagocytic signal CD24, establishing a “don’t eat me” cooperation
with M2-TAMs to evade the immune-killing effect. Moreover, considering the
crucial role of arginine in immune cell functions, competitive dynamics have emerged
among various immune cell types for this essential nutrient. M2 TAMs,
characterized by elevated Arg-1 expression, significantly deplete arginine,
thereby hindering the arginine-dependent activation of T cells and suppressing
their antitumor functions.
The aforementioned interaction patterns of cooperation and competition often
contribute to the overall tumor-promoting TME state. Consequently, targeting
this interaction based on metabolism-immune crosstalk provides a promising
therapeutic intervention for inhibiting tumor development. Nevertheless, the
complex cellular interaction network involving tumor and immune cells
demonstrates high flexibility and plasticity, necessitating coordinated
modulation across multiple cell types to reshape competitive-cooperative patterns.
Building on these concepts, we aimed to develop a delivery system that
integrates diverse regulatory modules while maintaining functional separation,
enabling precise modulation of distinct cellular states.
Outer membrane vesicles (OMVs) derived from
bacterial outer membranes represent potent immune response enhancers, harboring
antigens and pathogen-associated molecular patterns from bacteria. As intact nanoscaled
lipid bilayer vesicles, OMVs have been applied in various combined
immunotherapies. Fragmented OMVs have been reported to activate the immune
system via pathogen-associated molecular patterns. Drawing inspiration from
this, we proposed an OMV-based concept whereby internal cargo physicochemical
changes would induce OMV fragmentation and separation. This concept aimed to
utilize OMV fragments and their internal cargo to specifically target different
cell types in the TME, maximizing the harmonization of interactions among
immune cells and triggering
antitumor immune responses.
Therefore, we designed charge-reversal
nanocomplexes that are responsive
to acidic environments induced by abnormal
tumor metabolism and encapsulated them within engineered OMVs to form multifunctional multimodule intelligent
vesicles (charge-reversal polymer/siRNA@CD24
scFv-OMV/PTX nanocomplexes, CR/si@ab-OMV/PTX nanoparticles (NPs)). Triggered by
the reduced pH, the surface of the inside nanocomplexes becomes positive,
disrupting the outside vesicle
structure. The engineered OMVs were enriched with CD24
antibodies on the surface, which could navigate membrane fragments and hydrophobic chemotherapeutic drugs inserted in
them to tumor cells.
Consequently, tumor cells are killed, relieving metabolic stress in the TME and
affecting Arg-1 expression in TAMs. The exposed positive nanocomplexes specifically target M2 macrophages via modified mannose and deliver siRNAs to silence
Arg-1. TAMs
repolarized to the M1 type, and the "don't eat me" signal was blocked
by the antibodies, thereby enhancing macrophage phagocytosis of tumors. In
addition, due to the increase in L-arginine in the TME, the function of
effective T cells would be restored, forming an antitumor cooperative
relationship with
the repolarized TAMs in antitumor immunity. The activated immune system engages
in a survival competition with tumor cells, hereby suppressing tumor
proliferation. Collectively, the CR/si@ab-OMV/PTX NPs could achieve
simultaneous and specific delivery of multitarget and multifunctional modules
under simple conditions of reduced pH, demonstrating tremendous promise for reconciling
the competitive-cooperative patterns among malignant and immune cells and
activating antitumor immune response in the TNBC models.

Scheme 1. Schematic
illustration of reconciling the cooperative-competitive patterns among tumor
and immune cells of multi-module nanocomplexes (CR/si@ab-OMV/PTX NPs.)