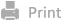Pleiotropic Microenvironment Remodeling Micelles for Cerebral Ischemia-Reperfusion Injury Therapy by Inhibiting Neuronal Ferroptosis and Glial Overactivation

Reperfusion injury presents a significant obstacle to neuronal survival
following successful recanalization in ischemic stroke, which is characterized
by intricate pathophysiological processes comprising numerous interconnected
pathways. Oxidative stress-induced neuronal ferroptosis and the overactivation
of glial cells play important roles in this phenomenon. In this study, we
developed a targeted cross-linked micelle loaded with idebenone to rescue the
ischemic penumbra by inhibiting neuronal ferroptosis and glial overactivation.
In rat models, the CREKA peptide-modified micelles accumulate in the damaged
brain via binding to microthrombi in the ipsilateral microvessels. Upon
reactive oxygen species (ROS) stimulation, diselenide bonds within the micelles
are transformed to hydrophilic seleninic acids, enabling synchronized ROS
consumption and responsive drug release. The released idebenone scavenges ROS,
prevents oxidative stress-induced neuronal ferroptosis, attenuates glial
overactivation, and suppresses pro-inflammatory factors secretion, thereby
modulating the inflammatory microenvironment. Finally, this micelle
significantly reinforces neuronal survival, reduces infarct volume, and
improves behavioral function compared to the control groups. The related
results, titled Pleiotropic Microenvironment Remodeling Micelles for
Cerebral Ischemia-Reperfusion Injury Therapy by Inhibiting Neuronal Ferroptosis
and Glial Overactivation, were published online in the ACS Nano, an
internationally renowned journal.
Cerebral ischemia-reperfusion injury (CI-RI) is a complex and multifaceted
pathophysiological process with several interconnected pathways that evolve
over time and affect multiple types of cells. Following reperfusion, excessive
reactive oxygen species (ROS) cause oxidative damage to DNA, proteins, and
lipids, resulting in neuronal cell death. While the dominant pathway of cell
death after reperfusion remains undetermined, emerging evidence suggests that
ferroptosis plays a crucial role in this injury. Ferroptosis is a newly
identified type of regulated cell death that relies on oxidative
stress-mediated production and accumulation of lipid peroxides, leading to
cytoplasmic membrane rupture and synchronous cell ferroptosis with extensive
tissue injury. Neurons undergoing ferroptosis release damage-associated
molecular patterns (DAMPs) that can rapidly activate and recruit microglia,
inducing the upregulation of NADPH oxidase and ROS production. In addition,
numerous research studies suggest that ROS can also act as proinflammatory
signaling molecules and contribute to the activation of microglia. Furthermore,
overactivated microglia secrete pro-inflammatory cytokines and chemokines that
attract astrocytes and peripheral leukocytes, exacerbating neuroinflammation
and oxidative stress. Ultimately, a vicious cycle of mutual promotion and
self-amplification is formed among oxidative stress, neuronal ferroptosis, and
inflammatory response, creating a lesion microenvironment detrimental to
neuronal survival. Previous studies have reported that the use of antioxidants
to decrease oxidative stress, ferroptosis inhibitors to suppress ferroptosis,
and anti-inflammatory agents to reduce neuroinflammation can all alleviate
reperfusion injury to a certain extent. These findings provide a foundation for
implementing pleiotropic therapeutics to remodel the complex microenvironment
to treat CI-RI.
In this study, we developed a CREKA peptide-modified IDBN-loaded
cross-linked polymeric micelle (CPLSeP/IDBN), intending to remodel the lesion
microenvironment to treat CI-RI. The micelles were assembled from a
ROS-responsive diselenide bond containing amphiphilic polymer and the
hydrophobic IDBN. CPLSeP/IDBN micelles exhibited enhanced solubility of IDBN
and a prolonged blood circulation time. By employing the surface-modified CREKA
peptide, the micelles demonstrated efficient binding to the microthrombus and
retention within the lesion microvessels. Upon arrival at the damaged brain
parenchyma through the disrupted BBB, the cross-linker was cleaved by ROS,
resulting in the consumption of ROS and simultaneous responsive drug release.
The released IDBN effectively reduced oxidative stress-induced neuronal
ferroptosis and attenuated the inflammatory response by inhibiting the
overactivation of glial cells, thereby achieving overall regulation of the
lesion microenvironment and exerting pleiotropic therapeutic effects (Scheme 1).
This study provides a promising approach for the targeted delivery of
pleiotropic therapeutics to remodel the complex postreperfusion
microenvironment to alleviate CI-RI.

Scheme 1. Illustration of
CPLSeP/IDBN Micelles Formation and Microenvironment Regulation in the Ischemic
Brain
Doctoral student Chao Li in
our group is the first authors of the paper, and Professor Jiang Chen is the
corresponding author of the paper. The research was supported by the National
Natural Science Foundation of China, the Shanghai Academic Research Leaders
Program and ZJLab.