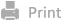Stimuli-Responsive Drug Delivery Systems Triggered by Intracellular or Subcellular Microenvironments

Drug
delivery systems (DDSs) triggered by local microenvironment represents the
state-of-art of nanomedicine design, where the triggering hallmarks at
intracellular and subcellular levels could be employed to exquisitely recognize
the diseased sites, reduce side effects, and expand the therapeutic window by
precisely tailoring the drug-release kinetics. Though with impressive progress,
the DDS design functioning at microcosmic levels is fully challenging and
underexploited. Here, we provide an overview describing the recent advances on
stimuli-responsive DDSs triggered by intracellular or subcellular
microenvironments. Instead of focusing on the targeting strategies as listed in
previous reviews, we herein mainly highlight the concept, design, preparation
and applications of intracellular models. Hopefully, this review could give
useful hints in developing nanoplatforms proceeding at a cellular level.

Scheme 1. Illustration on the stimuli-responsive drug delivery systems triggered by
intracellular or subcellular microenvironments
The
urgent demand, ironical phenomenon and stern reality urged us to seriously
rethink over the underlying logic mechanism, which is functioning
inadequately and fostering the dilemma. Scientists have made
great advancements and gained versatile skills in designing
nanoscale-structured materials and nanodevices, for circulatable, targetable
and penetrable nanomedicines to obtain more applicable approaches to enhancing
the therapeutic quality. These characteristics are also the main streams that
currently most pharmacists concerned. However, there is an irreconcilable (at
least, difficult) contradiction in the DDS design field that should be
seriously addressed: the intentionally high adhesion between the excipients and
drug molecules before arriving the targeted cell VS the demanded “burst-release” of drugs from DDS upon reaching the
aimed cell or organelles. Current design for drug delivery systems are mainly
aiming to resolve the physiological disposition before arriving/entering
targeted cells, including long circulation, high stability, optimized
targeting, fine distribution or deep penetration. It is truly tough for DDS
designers to both satisfy the contrary demands from two sides of the same coin,
with the aim of providing more accurate, more controllable and more reliable
protocols (besides versatility) in delivering the escorted drugs to the crucial
intracellular domain [8]. In other words, we should bring the attention on
intracellular/subcellular drug-release back to table, and focus a deeper
dimension at a cellular level, by designing/manufacturing sophisticated
nanostructures, and manipulating the DDS’ behavior according to the set program
even in the cells, since the final fate of nearly all drugs’ in vivo stories normally ends into the
target cells.
In
order to address the dilemma, the key factor is the determination of the
balance between stability and responsiveness of DDS, based on the profound
understanding of the intracellular or subcellular microenvironment (especially
of the pathological cells), molecular biological mechanism at a cell scale,
precise design of the dynamic chemical structures at a molecular level. The
perfection of combining the above can thus embody the key insight to structure
materials and scaffold useful devices as DDS by overcoming the accompanying
stability from circulation, in order to bring enormous immediate benefits in
the research and practice of on-demand drug-release at intracellular or
subcellular levels.
The
controllable, accurate and rational DDS, which can function as expected even at
the terminal intracellular stage of the whole drug-delivery process, is
undoubtedly an ultimate scientific challenge. The spatial-temporal profile of
up- or down-regulated intracellular hallmarks is quite tumor specific, and
could be adopted to selectively recognize the cancer cells by sensing the
abnormal conditions. In the past decade, scientists were attempting to realize
the on-demand drug-release systems at intracellular or subcellular levels,
which can detect and sense the particular intracellular biological signals, and
logically respond to the parameters accordingly to launch the escorted drugs at
the correct sites with suitable dose. Though there is still a long way to go to
fulfill the tasks, there are several DDS approaching the intracellular or
subcellular manipulation. In this review, we reviewed related progress by the
categorization of biological parameters, including intracellular pH,
glutathione (GSH), reactive oxygen species (ROS), hypoxia, enzymes, adenosine
triphosphate (ATP), and so on.
This
review, with Prof Tao Sun and Prof Chen Jiang as the first and corresponding
authors respectively, has been accepted by Adv Drug Deliv Rev, which can be
found via the link: https://www.sciencedirect.com/science/article/pii/S0169409X23000881?via%3Dihub