Microenvironment-tailored micelles restrain carcinoma-astrocyte crosstalk for brain metastasis

Based on the important role of the metastatic microenvironment on the colonization and development of metastatic tumors, our research group developed a microenvironment-regulated micelle to inhibit the crosstalk between brain metastases and astrocytes, and use chemotherapeutic drugs to kill tumor cells while correcting the metastatic microenvironment to achieve the treatment of brain metastases. The related results, titled microenvironment-tailored micelles restrain carcinoma-astrocyte crosstalk for brain metastasis, were published online in the Journal of Controlled Release, an internationally renowned journal.
The metastasis of malignant tumors to remote organs involves the spread of tumor cells and their adaptation to the tailored microenvironments of different metastatic sites and to co-evolve with the microenvironment. Surviving metastatic tumor cells can influence the surrounding normal cells through intercellular signaling, resulting in the formation of a pro-tumorigenic niche for tumor cell colonization, invasion, and growth. Breast cancer metastasizes to the lung, brain, liver, bone, and lymph, and 15-25% of the patients will develop brain metastases. Among breast cancers, triple-negative breast cancer (TNBC) exhibits the highest incidence of brain metastasis and the worst therapeutic outcomes (the median survival is only 4.9 months), and there is currently no effective clinical treatment.
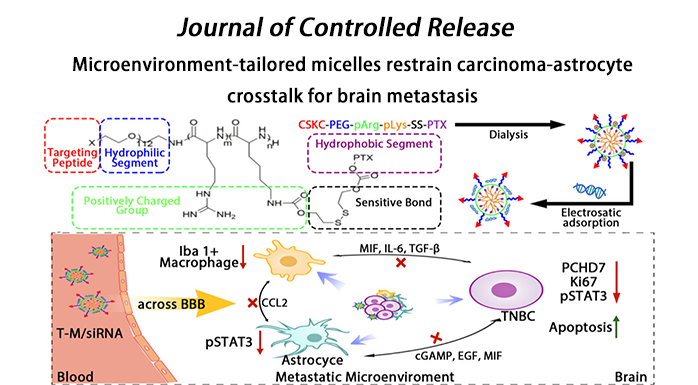
Upon the extravasation of breast cancer cells across the BBB into the brain, metastases can induce a strong local activation of astrocytes in the brain, resulting in the clearance of most metastatic cancer cells. The residual surviving metastatic cells can induce a shift in astrocyte phenotype—from a metastasis-inhibiting phenotype to a metastasis-promoting phenotype—and induce immunosuppressive activation of microglia, both of which depend on the crosstalk between the metastatic cells and astrocytes. As reported previously, the interactions between metastatic cells and astrocytes are not limited to cytokines; gap junctions—comprising Cx43 and PCDH7—are also formed as a crosstalk channel between the two cell types. Among the two gap junction proteins, PCDH7 is specifically expressed in brain metastatic cancer cells and is highly expressed on the surface of abnormally activated astrocytes around the brain metastasis. Downregulation of PCDH7 in TNBC has been reported capable of inhibiting brain metastasis progression and chemoresistance generation. Therefore, normalizing the brain metastasis microenvironment via PCDH7 silencing to inhibit the gap junction formation and eliminating brain metastatic cancer cells by chemotherapeutic delivery improve the treatment outcomes of patients with brain metastasis. Even so, it is difficult to enable the accumulation of siRNA with microenvironmental correction function at the site of metastasis because of their rapid metabolism. Owing to the BBB barrier effect, siRNA and chemotherapeutics (such as PTX, the first-line drug for breast cancer treatment) cannot be efficiently delivered to the brain regions harboring a high proportion of multiple brain metastases with scattered lesions; this results in their therapeutic effects being severely limited. Recent studies on co-delivery of paclitaxel and siRNA involve chemotherapy and microenvironment regulation, chemotherapy and resistance reversal, chemotherapy and anti-angiogenesis, etc. However, due to the challenges posed by the special brain environment, only limited studies based on the combination strategy have been reported to ameliorate the brain metastatic therapy. Previous study placed a greater impetus on improving drug delivery rather than normalizing the brain metastatic microenvironment. In summary, it is important to design an efficient targeted drug delivery nanoplatform aimed at normalizing the metastatic microenvironment and eliminating metastatic cancer cells.
In this study, we constructed T-M/siRNA with high systemic stability that released PTX and siRNA into the cytosol in response to GSH to improve the outcomes of breast-to-brain metastatic therapy. T-M/siRNA can effectively cross the BBB and target TNBC brain metastatic cancer cells through the anchored D-type CSKC cyclic short peptide. The disulfide bonds of T-M/siRNA are cleaved in the presence of high GSH level, resulting in PTX-release and induction of micellar dissociation. Consequently, the charge density of the complexes would be lowered to accelerate the release of siRNA against PCDH7, resulting in gene silencing. We found that T-M/siRNA could impair the carcinoma-astrocyte crosstalk, inhibit the activation of the signal transducer and activator of transcription 3 (STAT3) signaling pathway and reduce the recruitment of Iba1+ microglia/macrophages, thereby significantly ameliorating treatment compared to that observed upon employing a single chemotherapy strategy in a mouse model of TNBC (BrM-bearing mice).
Doctoral students Zhao Zhenhao and Zhang Yujie in our group are the co-first authors of the paper, and Professor Jiang Chen is the corresponding author of the paper. The research was supported by the National Natural Science Foundation of China, the Shanghai Academic Research Leaders Program and other projects.
For more information: https://doi.org/10.1016/j.jconrel.2022.07.009